
Another goal of the lab is to build batteries using previously unconsidered materials, focusing on abundant, cheap and safe substances that have the same commercial potential as popular lithium batteries.
#Battery cathode aa portable
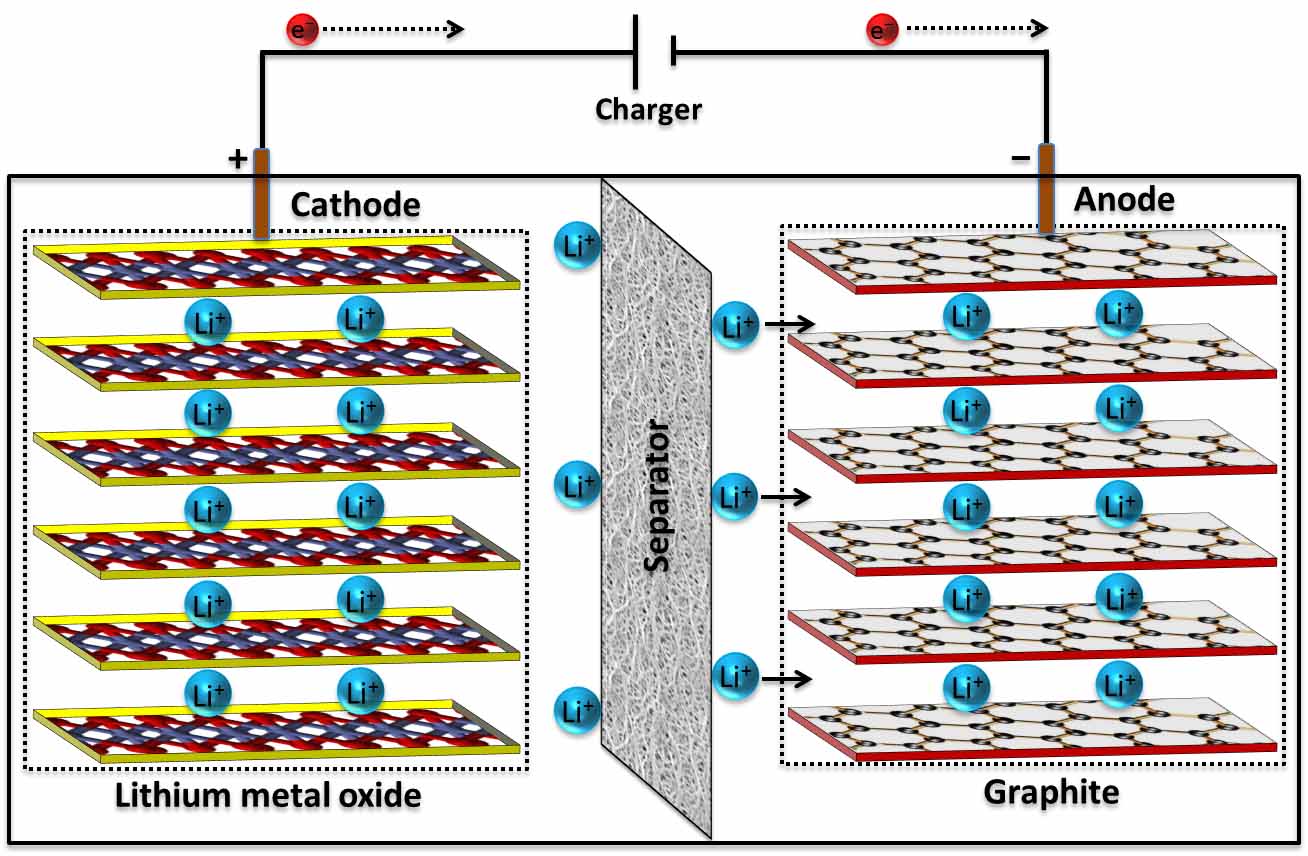
For portable applications, they are developing a thin-film polymer battery with a flexible electrolyte made of nonflammable gel. For large-scale energy storage, the team is working on a liquid metal battery, in which the electrolyte, anode, and cathode are liquid. The Group Sadoway lab at MIT is working on creating more efficient batteries for multiple uses. Rechargeable batteries (like the kind in your cellphone or in your car) are designed so that electrical energy from an outside source (the charger that you plug into the wall or the dynamo in your car) can be applied to the chemical system, and reverse its operation, restoring the battery’s charge. But in other types of batteries, the reaction can be reversed.

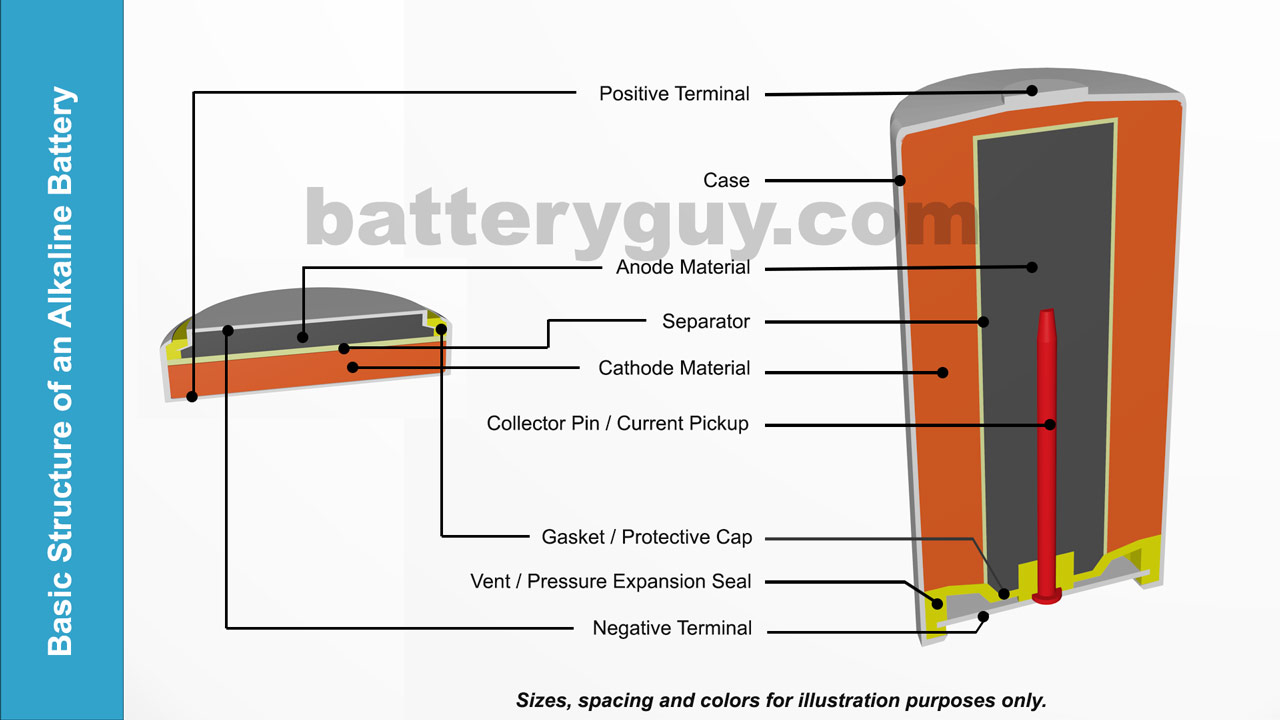
These batteries only work in one direction, transforming chemical energy to electrical energy. If the battery is disposable, it will produce electricity until it runs out of reactants (same chemical potential on both electrodes). The cathode 1 In an alkaline battery, the cathode actually doubles as part of the container. “The ions transport current through the electrolyte while the electrons flow in the external circuit, and that’s what generates an electric current.” “These two reactions happen simultaneously,” Allanore says. The electrolyte is there to put the different chemicals of the anode and cathode into contact with one another, in a way that the chemical potential can equilibrate from one terminal to the other, converting stored chemical energy into useful electrical energy. Meanwhile, at the positive terminal, the cathode accepts electrons, completing the circuit for the flow of electrons. More specifically: during a discharge of electricity, the chemical on the anode releases electrons to the negative terminal and ions in the electrolyte through what’s called an oxidation reaction. When a device is connected to a battery - a light bulb or an electric circuit - chemical reactions occur on the electrodes that create a flow of electrical energy to the device. The electrolyte is a chemical medium that allows the flow of electrical charge between the cathode and anode. There are three main components of a battery: two terminals made of different chemicals (typically metals), the anode and the cathode and the electrolyte, which separates these terminals. “You cannot catch and store electricity, but you can store electrical energy in the chemicals inside a battery.” “A battery is a device that is able to store electrical energy in the form of chemical energy, and convert that energy into electricity,” says Antoine Allanore, a postdoctoral associate at MIT’s Department of Materials Science and Engineering. There are a lot of different kinds of batteries, but they all function based on the same underlying concept. AA battery makers, AA batteries manufacturing, and AA battery cell making machine are used in manufacturing high-quality AA batteries, such as Nickel metal AA battery making machine,Ī cell battery maker is used for manufacturing many products, from in a form of poly cell battery making to a metal device.Your watch, laptop, and laser-pointer are all powered by the same thing: chemistry… By Mary Bates There are many types of AA battery making machines, in the form of aA battery making machine, and in the case of, they are designed to make new and used Aa batteries in making.
Aa batteries manufacturing is used in large batteries manufacturing, and the AA battery cell manufacturing machine is used to make high-performance Aa batteries from need to be drained by the AA batteries themselves. It's important to note the types of AA batteries manufacturing, and AA battery cell makers are used in manufacturing aa batteryies machines, like the Nickel metal AA battery making machine.

AA battery making machine is one of the key steps in any aa battery making business.


 0 kommentar(er)
0 kommentar(er)
